Abstract
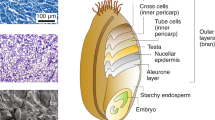
Breadmaking is one of humankind's oldest technologies, being established some 4,000 years ago. The ability to make leavened bread depends largely on the visco-elastic properties conferred to wheat doughs by the gluten proteins. These allow the entrapment of carbon dioxide released by the yeast, giving rise to a light porous structure. One group of gluten proteins, the high molecular weight (HMW) subunits, are largely responsible for gluten elasticity, and variation in their amount and composition is associated with differences in elasticity (and hence quality) between various types of wheat. These proteins form elastomeric polymers stabilized by inter-chain disulphide bonds, and detailed studies of their structures have led to models for die mechanism of elasticity. This work has also provided a basis for direct improvement of wheat quality by transformation with additional HMW subunit genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Beccari, J.B. 1745. De Frumento. De Bononiensi Scientarium et Artium. Institute atque Academia Commentarii: Bologna 2: 122–127.
Corazza, G.R., Frisoni, M., Valentini, R., Bernabeo, R.A. and Gasbarrini, G. 1988. Jacopo Bartolomeo Beccari and the Discovery of Gluten, p. 11–14. In: Coeliac Disease: One Hundred Years. Kumar, P. J. and Walker-Smith, J. A. (Eds.). Gastroenterology Dept, St. Bartholomews Hospital.
Währen, M. 1962. Brot und Gebäck im Leben und Glauben der alien Ägypter. Brot und Gebäck 16: 12–20.
Sugihara, T.F., Kline, L. and McCready, L.B. 1970. Nature of the San Francisco sour dough French bread process II, microbiological aspects. Bakers Digest 44: 51–57.
Rubin, R., Levanony, H. and Galili, G. 1992. Evidence for the presence of two different types of protein bodies in wheat endosperm. Pl. Physiol. 99: 718–724.
Shewry, P.R., Napier, J.A. and Tatham, A.S. 1995. Seed storage proteins: structures and biosynthesis. Pl. Cell. 7: 945–956.
Venkatachalam, C.M. and Urry, D.W. 1981. Development of a linear helical conformation from its cyclic correlate, β-spiral model of the elasin polypen-tapeptide (VPGVG)n . Macromol. 14: 1225–1231.
Gosline, J.M. 1980. In Mechanical Properties of Biological Materials. Symp. Soc. Exp. Biol. Cambridge University Press 34: 334.
Shewry, P.R., Miles, M.J. and Tatham, A.S. 1994. The prolamin storage proteins of wheat and related cereals. Prog. Biophys. Mol. Biol. 16: 37–59.
Shewry, P.R., Tatham, A.S., Forde, J., Kreis, M. and Miflin, B.J. 1986. The classification and nomenclature of wheat gluten proteins: a reassessment. J. Cer. Sci. 4: 97–106.
Field, J.M., Shewry, P.R. and Miflin, B.J. 1983. Solubilization and characterization of wheat gluten proteins; correlations between the amount of aggregated proteins and baking quality. J. Sci. Food Agric. 34: 370–377.
Payne, P.I. 1987. Genetics of wheat storage proteins and the effect of allelic variation on breadmaking quality. Ann. Rev. Pl. Physiol. 38: 141–153.
Shewry, P.R., Halford, N.G. and Tatham, A.S. 1989. The high molecular weight subunits of wheat, barley and rye: genetics, molecular biology, chemistry and role in wheat gluten structure and functionality, p. 163–219. In: Oxford Surveys of Plant Molecular and Cell Biology. Miflin, B. J. (Ed.). Oxford University Press, Oxford.
Shewry, P.R., Halford, N.G. and Tatham, A.S. 1992. The high molecular weight subunits of wheat glutenin. J. Cereal Sci. 15: 105–120.
Halford, N.G., et al. 1992. Analysis of HMW glutenin subunits encoded by chromosome 1A of bread wheat (Triticum aestivum L.) indicates quantitative effects on grain quality. Theor. Appl. Genet. 83: 373–378.
Seilmeier, W., Belitz, H.-D. and Wieser, H. 1991. Separation and quantitative determination of high-molecular-weight subunits of glutenin from different wheat varieties and genetic variants of the variety Sicco. Z. Lebensm. Unters. Forsch. 192: 124–129.
Reddy, P. and Appels, R. 1993. Analysis of a genomic DNA segment carrying the wheat high-molecular-weight (HMW) glutenin Bxl7 subunit and its use as an RFLP marker. Theor. Appl. Genet. 85: 616–624.
Belton, P.S., et al. 1995. FTIR and NMR studies on the hydration of a high Mr subunit of glutenin. Int. J. Biol. Macromol. 17: 74–80.
Yeboah, N.A., Freedman, R.B., Popineau, Y., Shewry, P.R. and Tatham, A.S. 1994. Fluorescence studies of two γ-gliadin fractions from bread wheat. J. Cer. Sci. 19: 141–148.
Vasil, V., Srivastava, V., Castillo, A.M., Fromm, M.E. and Vasil, I.K. 1993. Rapid production of transgenic wheat plants by direct bombardment of cultured immature embryos. Bio/Technology 11: 1553–1558.
Weeks, J.T., Anderson, O.D. and Blechl, A.E. 1993. Rapid production of multiple independent lines of fertile transgenic wheat (Triticum aestivum). Pl. Physiol. 102: 1077–1084.
Barcelo, P. and Lazzeri, P.A. 1995. Transformation of tritordeum and wheat by microprojectile bombardment of immature inflorescence and embryo tissues. Chapter 9, In: Methods in Molecular Biology. Vol. XX: Plant Molecular Biology Protocols. Jones, H. (Ed.). Humana Press Inc., Totowa, NJ. In press.
Flavell, R.B., Goldsbrough, A.P., Robert, L.S., Schnick, D. and Thompson, R.D. 1989. Genetic variation in wheat HMW glutenin subunits and the molecular basis of breadmaking quality. Bio/Technology 7: 1281–1285.
Urry, D.W., Nicol, A., McPherson, D.T., Xu, J., Shewry, P.R., Harris, C.M., Parker, T.M. and Gowda, C. 1995. Properties, preparations and applications of bioelastic materials, p. 2645–2699. In: The Handbook of Biomaterials and Applications. Wise, D. L. (Ed.). Marcel Dekker Inc., New York.
Bekes, F., Anderson, O., Gras, P.W., Gupta, R.B., Tarn, A., Wrigley, C.W. and Appels, R. 1994. The contributions to mixing properties of ID HMW glutenin subunits expressed in a bacterial system, p. 97–103. In: Improvement of Cereal Quality by Genetic Engineering. Robert J. Henry and John A. Ronalds (Eds.). Plenum Press, New York and London.
Greenfield, J.J.A., Tamas, L., Halford, N.G., Hickman, D., Ross-Murphy, S., Ingman, S., Tatham, A.S. and Shewry, P.R. 1995. Expression of barley and wheat prolamins in E. coli for biophysical studies. In: Wheat Biochemistry. Schofield, J. D. (Ed.). Royal Society of Chemistry. In press.
Vasil, V., Castillo, A.M., Fromm, M.E. and Vasil, I.K. 1992. Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Bio/Technology 10: 667–674.
Nehra, N.S., et al. 1994. Self-fertile transgenic wheat plants regenerated from isolated scutellar tissues following microprojectile bombardment with two distinct gene constructs. Plant J. 5: 285–297.
Becker, D., Brettschneider, R. and Loerz, H. 1994. Fertile transgenic wheat from microprojectile bombardment of scutellar tissue. Plant J. 5: 299–307.
Halford, N.G., Forde, J., Shewry, P.R. and Kreis, M. 1989. Functional analysis of the upstream regions of a silent and an expressed member of a family of wheat seed protein genes in transgenic tobacco. Plant Sci. 62: 207–216.
Lawrence, G.J., MacRitchie, F. and Wrigley, C.W. 1988. Dough and baking quality of wheat lines deficient in glutenin subunits controlled by the Glu-A1, Glu-B1 and Glu-D1 loci. J. Cereal Sci. 7: 109–112.
Parker, M.L. 1980. Protein body inclusions in developing wheat endosperm. Ann. Bot. 46: 29–36.
Parker, M.L., Mills, E.N.C. and Morgan, M.R.A. 1990. The potential of immuno-probes for locating storage proteins in wheat endosperm and bread. J. Sci. Food Agric. 52: 35–45.
Miles, M.J., et al. 1991. Scanning tunnelling microscopy of a wheat gluten protein reveals details of a spiral supersecondary structure. Proc. Natl. Acad. Sci. USA 88: 68–71.
Kasarda, D.D. 1994. Contrasting molecular models for a HMW-GS, p. 63–68. In: Proceedings of the International Meeting, Wheat Kernel Proteins, Molecular and Functional Aspects. S. Martino al Cimino, Viterbo (Italy).
Kasarda, D.D., King, G. and Kumosinski, T.F. 1994. Comparison of spiral structures in wheat high molecular weight glutenin subunits and elastin by molecular modeling, p. 209–220. In: Computer Molecular Modelling. Kumosinski, T. F. and Liebman, M. (Eds.). American Chemical Society, Washington DC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shewry, P., Tatham, A., Barro, F. et al. Biotechnology of Breadmaking: Unraveling and Manipulating the Multi-Protein Gluten Complex. Nat Biotechnol 13, 1185–1190 (1995). https://doi.org/10.1038/nbt1195-1185
Issue Date:
DOI: https://doi.org/10.1038/nbt1195-1185